GOOD HEALTH FOR EVERYONE.
EXPLORE OUR BUSINESS
Elevating Healthcare through High-Quality APIs
At Synthimed Pharma, our primary business focus revolves around crafting high-quality Active Pharmaceutical Ingredients (APIs) with the noble objective of promoting good health for all. Originating as an API manufacturer, we have diligently cultivated this business domain, cementing it as a cornerstone of our organizational strength. Today, we proudly stand as one of the top API manufacturers in India, a testament to our unwavering dedication to advancing the production of essential pharmaceutical ingredients for the betterment of global healthcare.
Explore Our Verticals
From our modest origins, we have expanded our product range to encompass a wide array of segments, catering to a robust customer base across the globe. Renowned as a trusted API partner for numerous prominent organizations in both generic and innovative domains, we boast a proven track record, unwavering quality, and a broad therapeutic range, all bolstered by our world-class manufacturing facilities and regulatory expertise.
Our product list covers complex and generic APIs that require to be manufactured in dedicates manufacturing areas. Our organization go for fliling a large of DMFs every year based upon the technologies developed in our R&D. Our Manufacturing facilities mark approvals and certifications from global regulatory agencies including USFDA, EDQM, ANVISA and KFDA among others.
Our R&D is sufficed with advanced technical and chemistry capabilities. The state of art Research Centre spread over the area of 40,000 sq.ft. is engaged in developing process technology for APIs in different therapeutic categories. Our team has so far developed more than 40 APIs leading to fillings of DMFs with several Regulatory authorities. Our enthusiastic & experienced team of scientists has been developing novel multi step regulatory compliant processes in shortest possible time span. Our CRAMS offering ensure end to end support to our clients.
APIs
At Synthimed, our primary commitment is to craft top-tier Active Pharmaceutical Ingredients (APIs) with a steadfast objective of promoting universal health and well-being. Originally established as an API manufacturer, we have honed and fortified this business domain, evolving into one of India’s foremost API manufacturers.
CRAMS
Today, Synthimed is one of India’s leading API and Contract Research and Manufacturing Services provider supporting R&D programs from process development.
Impurities
Synthimed offers reference standards and impurities for over 25 APIs. Our Impurity portfolio is stocked with a wide range of standards for quick and reliable supply.
Research & Development
Our R&D center is primarily focused on the development and optimization of new and existing process technologies supporting the manufacturing of Active Pharmaceutical Ingredients (APIs).
High-Quality Active Pharmaceutical Ingredients (APIs) for Global Well-being
At the core of our business focus lies the creation of top-notch Active Pharmaceutical Ingredients (APIs) aimed at fostering good health for all. Having originated as an API manufacturer, we have diligently cultivated this sector, establishing it as a cornerstone of our expertise over many years. Today, we take immense pride in our standing as one of the premier API manufacturers in India.
While our API business predominantly caters to the demands of regulated markets worldwide, including the USA, Europe, Korea, Japan, and Latin America, it’s our profound technical acumen in this field that has underpinned our success. Our unwavering command over Intellectual Property Rights (IPR) and Regulatory Affairs has consistently enabled us to deliver products that meet the global standards for quality and regulation, including cGMP and cGLP, thus contributing to our continued success and leadership in the industry.


CRAMS
Synthimed Labs Private Limited stands as one of India’s foremost API and Contract Research and Manufacturing Services providers, offering comprehensive support for R&D programs from process development to clinical supplies. The company’s diverse expertise encompasses synthetic medicinal chemistry, custom synthesis, process R&D, cGMP manufacturing, analytical development, and regulatory support. Synthimed Labs Private Limited boasts state-of-the-art research facilities with USFDA, KFDA, TGA, WHO GMP, PMDA, and EDQM approved manufacturing units, including an isolated HPAPI manufacturing facility with an OEL of > 0.3 μg/m3/ 8/hr.
Synthimed Labs Private Limited caters to the synthetic chemistry aspect of both early and late-phase drug development and is reinforced by a robust scientific and project management team that ensures the punctual execution and delivery of projects. Through its scientific acumen, cost competitiveness, and adherence to best practices in confidentiality and protection of intellectual property, Synthimed Labs Private Limited consistently delivers substantial value to its global clientele
Spectrum of Services
Synthimed Labs provides a wide range of synthetic chemistry services ranging from discovery through the development and manufacture of small molecules as seen below.
Discovery Services
Library Synthesis & Reference Cpds
Synthesis of Analogs
Scaffold Synthesis
Medicinal Chemistry Support

Manufacturing Services
(Clinical Supplies- Early & Late Phase)
APIs and Intermediates
Veterinary APIs
Specialty Molecules including HPAPIs
Custom Chemical Synthesis

Development Services
Route Scouting
Process Development and Quality by Design
Drug Substance Development

Analytical/Stability Services
Method Development, Validation & Transfer
Impurity Profiling, Isolation, Identification & Characterization

* All Discovery and Developmental Services are offered either on a Fee For Service (FFS) or Full Time Equivalent (FTE) basis.
Industries We Serve
Synthimed Labs Private Limited caters to the research, development, and manufacturing requirements of a variety of industry sectors including:

Pharmaceutical

Biotechnology

Animal Health
Agrochemicals

Academic Research

Specialty Chemicals
Chemistry Services/Capabilities
Synthimed Labs offers a number of services in the synthetic chemistry area involving process development and scale-up of simple and complex heterocyclic, carbocyclic as well as chiral molecules.
Medicinal Chemistry
Synthimed Labs has developed special expertise in synthetic medicinal chemistry in route scouting, support in lead optimization, and synthesis of drug metabolites.
Library Synthesis
Synthimed Labs has a solid track record of delivering library sets in collaboration with clients in the healthcare sector. Our collaborations in library synthesis are mostly on a FTE business model.
Material Sciences
Synthimed Labs has also developed unique expertise in offering a wide range of services in the synthetic chemistry area supporting the development of compounds used in Organic Light Emitting Diodes (OLED) containing diverse heterocyclic frameworks. Specifically, transition metal catalyzed C-C cross coupling involving applications of Grignard, Suzuki, Negishi, Heck, Stille, Kumada reactions are extensively exploited on commercial scale to synthesize high purity OLED precursors and other chemicals.
Reaction Capabilities
- Grignard
- Chiral Reduction
- Chemical Resolution
- Biotransformations
- Organometallic
- Organoborane Chemistry
- Palladium Chemistry
- Cyanation
- Chlorination
- Bromination
- Nitration
- Reductive Amination
- Catalytic Hydrogenation
- LAH Reduction
- Condensation
- Cyclization
- Friedel Crafts
- Diazotization
- Wittig
- Mesylation
- Methylation
- Demethylation
- Dehydrogenation
- Gewald Reaction
- Oxidation of Sulfide
Manufacturing Capabilities
Synthimed Labs has the following sites available for catering to the customer requirements.
Dedicated Kilo lab at R&D center, Mohali, India
- All Glass Assembly reactors : 20 L, 50 L, 100 L
- Temperature range : – 60 °C to +180 ° C
- SS 316 Hydrogenator : 2 L, 25 L, Pressure upto 25 kg/cm2 and Temperature range – 5 ° C to +115 ° C
- Classified area for intermediates and API’s to run parallel reactions and projects
- Necessary supporting equipments like filtration, drying, sieving, milling and micronisation in Class 100,000 area


Manufacturing Unit 1: Dera Bassi, India (API production facility)
- Over 536 KL of reaction volume consisting of over 200 Glass assemblies and SS 316 reactors with capacity ranging from 0.02KL to 25KL
- Temperature range of – 80 ° C to +180 ° C
GMP Pilot Plant at Unit 1: Dera Bassi, India (Suitable for advance Intermediates , KSM, RSM, API)
- Over 4.0 KL of reaction volume consisting of SS 316 & GLR reactors and Glass assemblies
- Temperature range of – 80 ° C to +180 ° C
- Capacity ranging from 20 L to 1000 L


Hydrogenation Plant at Unit 1: Dera Bassi, India
- Capacity : 1500 L, 1200 L, 600 L, 35 L
- Temperature range: – 5 ° C to +115 ° C
- Pressure upto 20 kg/cm
Manufacturing Unit 2: Jammu, India
(Suitable for Intermediate & API)
-
Over 90.0 KL of reaction volume consisting of GL & SS 316 reactors


All manufacturing facilities are well supported with appropriate:
- Filtration Equipments: Centrifuge, Nutsche filters
- Post Production Equipments: Steam tray Dryer, Vacuum tray Dryer, Multimill, Sifter, Micronizer, Blenders
- Supporting Utilities
- Binary and Ternary solvent recovery plants
- QC equipped with modern Analytical equipments
High Potent – API Facility
API Manufacturing Facility located in Unit-1, Derabassi
- Approved cGMP facility, Class 100,000 cytotoxic suites
- Occupational exposure limit (OEL) < 0.3 μg/m3/ 8 h
- Suitable for handling Safebridge category – 3 products
- Containment through AHU, Pressure Zoning, Isolators and Personal Protective Equipment
- Isolators equipped with HEPA filters in supply & double HEPA filters at exhaust
- Isolators with negative pressure to avoid contamination and exposure
- Material flow through Pass boxes
- Accreditations from TGA Australia, WHO GMP, USFDA & EDQM
Equipment and Infrastructure
- Reactors
- SS316: 1 x 1.5 KL, 2 x 0.25 KL, 1 x 1.0 KL
- MSGL: 2 x 0.25 KL
- GA : 1 x 20 L
- SS316 Isolator : 7
- Column chromatography
Filtration Equipments
- Centrifuges, sparkler filters
Supporting post production Equipments
- Vacuum tray dryers, Microniser, Multimill, Sifters, Blenders
- Operating temperature range: – 15 ° C to +130 ° C
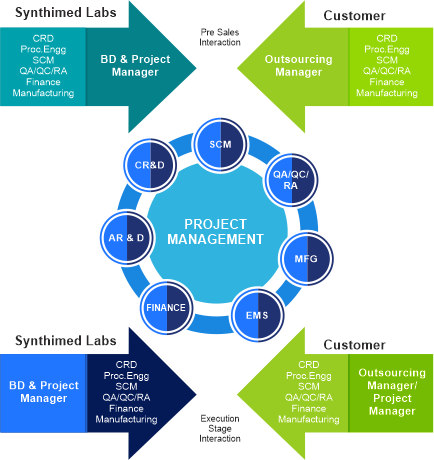
Project Management
Synthimed Labs employs a strong project management team that is responsible for cross functional coordination, project planning and liaising with all stake holders. The project manager acts as a single point of contact for all pre-sales interactions, non- technical aspects of the project including project kick-off and closure meetings, license procurement and allocating the necessary resources required for smooth execution of the project. The project manager also coordinates closely with the technical leads on scope changes, additional resource requirements and tracking all activities of the project.
REFERENCE STANDARDS / IMPURITIES
Synthimed offers reference standards and impurities for over 25 APIs. Our impurity portfolio is stocked with a wide range of standards for quick and reliable supply. Synthimed understands the industry’s need for quality and reliability and thus all the impurities we produce are as per current regulatory norms. All impurities are shipped with full documentation including; a Certificate of Analysis and MSDS to ensure smooth custom clearance.
RESEARCH AND DEVELOPMENT
Cutting-Edge Research and Development Facility at Synthimed
Synthimed is home to a cutting-edge Research and Development center spanning over 40,000 sq. ft. in Mohali (Punjab). This facility is dedicated to the advancement and refinement of new and existing process technologies crucial to the production of Active Pharmaceutical Ingredients (APIs).
Our R&D center is staffed with a highly skilled team of scientists hailing from leading global institutes. This accomplished team has successfully developed over 50 APIs spanning various therapeutic areas, showcasing our commitment to innovation and excellence in pharmaceutical research and development.


The current API process development protocol extensively involves principles of quality by design (QbD), design of experiments (DOE) and risk analysis so that robust and cost-effective processes are established.
R&D has developed and commercialized process for over 50 complex APIs with Macrolide Antibiotics as our major area of interest.
Special focus is on developing safe and effective processes that are environment friendly.
Protection of Intellectual Property on products developed for various countries.

